
Gravimetric Sorption Analysis
Gravimetric Sorption Analyzer from high vacuum up to 350 bar
Linseis GSA-Series
Linseis offers a wide range of measuring devices for gravimetric sorption analysis (GSA).
Our integrated solutions offer the possibility to control the pressure, temperature and gas dosing independently of each other using the supplied software.
We offer two different load cells, a mechanical microbalance and a magnetic levitation balance.
The magnetic levitation balance offers a unique, hermetically sealed measuring cell for highly reactive gases.
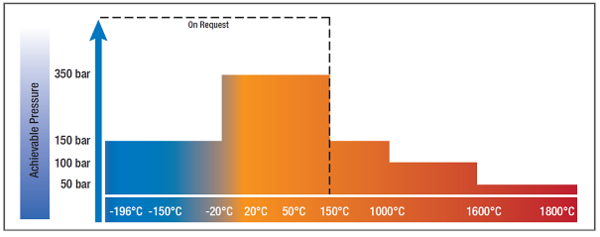
There is therefore a large selection of available devices in a temperature range from -196 °C to 2400 °C and a pressure range from ultra-high vacuum to 350 bar.
An optional TG-DSC (Thermogravimetry – Differential Scanning Calorimetry) measuring head enables the simultaneous determination of weight changes and caloric reactions in one measuring run.
Linseis offers the widest range of sorption analyzers for numerous applications such as
- Chemisorption/physisorption (UHV up to 350 bar)
- Adsorption isotherms
- TPD, TPO, TPR measurements (-196 to 1800°C)
- Sorption enthalpies (simultaneous TG/DSC sensor)
- In-situ gas analysis (FTIR, Raman, MS)
- Corrosive atmospheres Magnetic levitation balance
Gravimetric sorption
The adsorption or desorption of various gases by materials such as catalysts or porous structures is a special but very frequently requested application on thermobalances.
In general, there are two ways to monitor adsorption and desorption, volumetric sorption, where a certain amount of gas is introduced into a sample chamber and the change in pressure provides the information, and gravimetric sorption, where the change in mass of the sample is analyzed.
In our thermobalance series, we use the gravimetric sorption arrangement made possible by our high-resolution load cells, which can monitor very small mass changes over a wide pressure range.
This allows the device to precisely analyze the adsorption and desorption behavior of various materials in a temperature range from -196°C to 2400°C and a pressure range from 0.01 mbar to 350 bar.
Gravimetric sorption has many advantages: If a DSC measuring system is used, it is also possible to record the sorption enthalpy during the measurement, which can also provide very interesting information about the adsorption process.
Another point is the flexibility in measuring systems and sample volumes, which can be a few milligrams or even a few grams with different sample holders.
Gravimetric sorption is the simpler, faster and more flexible method to obtain information about gas adsorption, even if the accuracy of volumetric sorption cannot be achieved so far.
The following devices can be used for gravimetric sorption analysis:
- GSA PT 10 (STA PT 1600)
- GSA PT 100 (MSB)
- GSA PT 1000 (STA HP)
Areas of application:
- Gas sorption analysis
- Sorption enthalpies (simultaneous TG/DSC sensor)
- Density determinations
- Gas storage
- Zeolite
- Catalysts
- In-situ gas analysis (FTIR, Raman, MS)
- Kinetic analysis
- Corrosive atmospheres – Magnetic suspension balance
- Adsorption isotherm (BET Surface Analysis)
- TPD, TPO, TPR measurements (-196°C to 1800°C)
FTIR Coupling for GSA
All LINSEIS gravimetric sorption systems can be coupled with additional analysis tools such as FTIR.
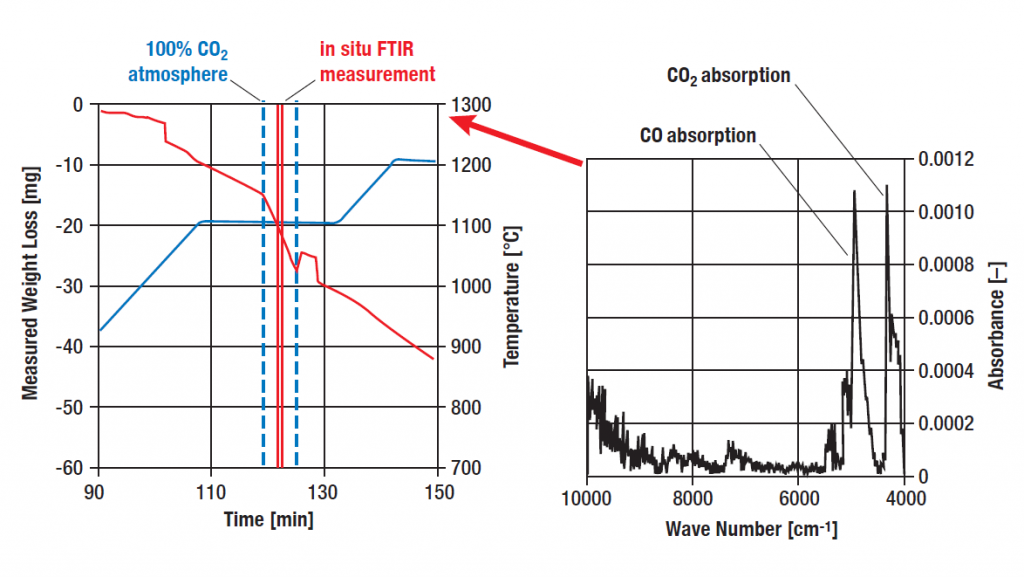
In the experiment, a charcoal sample was gasified in a CO2 atmosphere.
For this purpose, the charcoal sample was placed in the device and the temperature was increased at constant CO2 pressure.
During an isothermal segment at 1100°C, an enormous mass loss was observed, symbolizing the gasification of the bound carbon (according to the equation C+CO2 2 CO).
The graph shows the in-situ FTIR measurement during the gasification of coal in a TGA system. (Sample temperature: 1100°C, pure CO2 atmosphere at a gas flow rate of 20 ml/min at 273K, 0.013 bar)
The FTIR measurement was performed during the main mass loss step for 30 s.
The CO and CO2 signals are clearly visible in the IR spectrum, which proves the release of CO gas during mass loss.
Are you interested in a GSA measuring device?
Would you like to carry out a
sample measurement?
Contact us today!

Thomas
Phone: +1 (609) 223 2070
+49 (0) 9287/880 0
[email protected]
Quicklinks
Reach your goal quickly
Well informed
Downloads
Everything at a glance
Contact form
How new materials have been steadily improving our quality of life
for centuries.
Use the quotation form to send us a specific request for a quotation.







