Description
To the point
During heating, samples often undergo phase transitions and/or weight changes due to the evaporation of solvents and/or chemical reactions. These changes can be detected by thermal analysis: Calorimetric methods (DTA and DSC) provide information about the heat generated during these processes and thermogravimetry (TGA) shows the change in weight.
A change in weight can either be an increase in weight due to oxidation reactions or a loss in weight due to decomposition caused by the release of volatile compounds. Analysis of these evolved gases can provide valuable information about the composition of the sample and the reaction pathways for decomposition. Since thermal analysis does not provide information about the nature of the evolved gases, coupling with spectrometers or chromatographs is a valuable tool for analyzing evolved gases (EGA).
Unique features

Combined thermal analysis
and GC-MS:
For detailed analysis of the decomposition gases released
.
High detection sensitivity:
Detection and identification
of small gas volumes.
Versatile applications:
Suitable for chemical
reaction studies and
material analyses.
Direct coupling:
Easy integration with
thermobalances and other
thermal analyzers.
Wide temperature range:
Supports measurements at
high temperatures.
Questions? Just give us a call!
+49 (0) 9287/880 0
Thursday from 8 am to 4 pm
and Friday from 8 am to 12 pm.
We are here for you!
Application
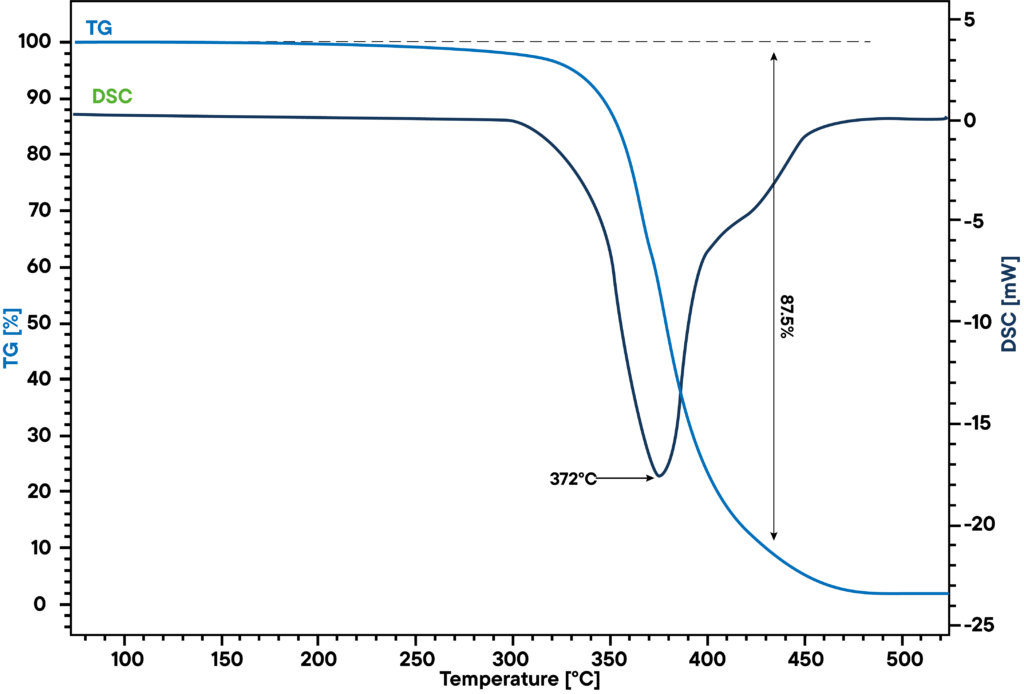
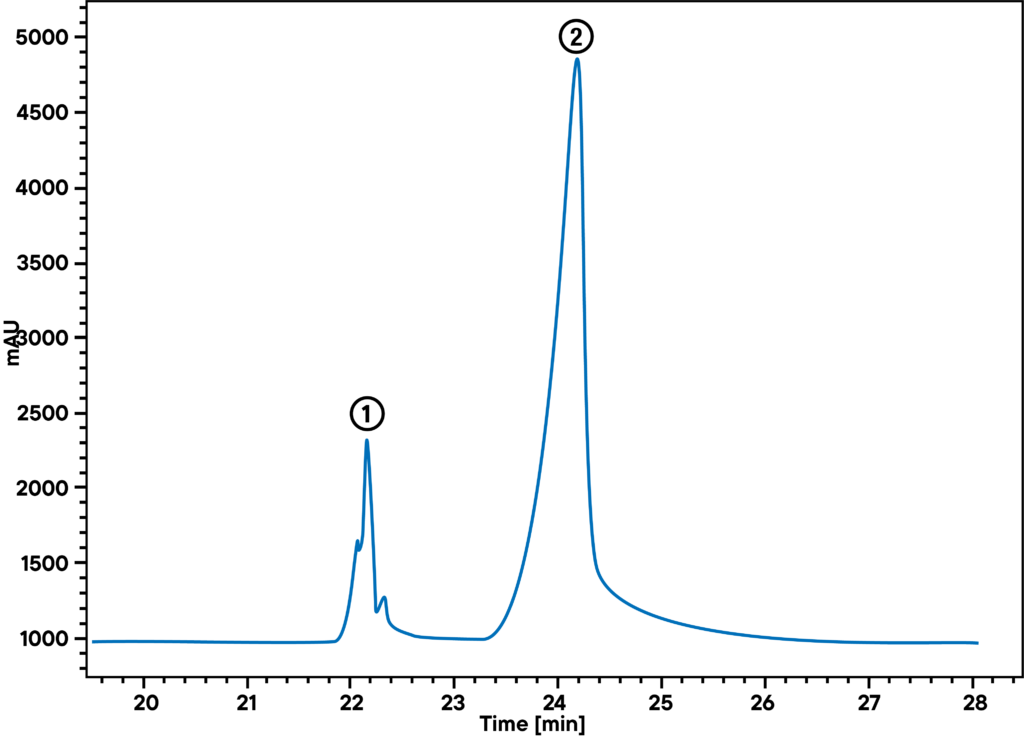
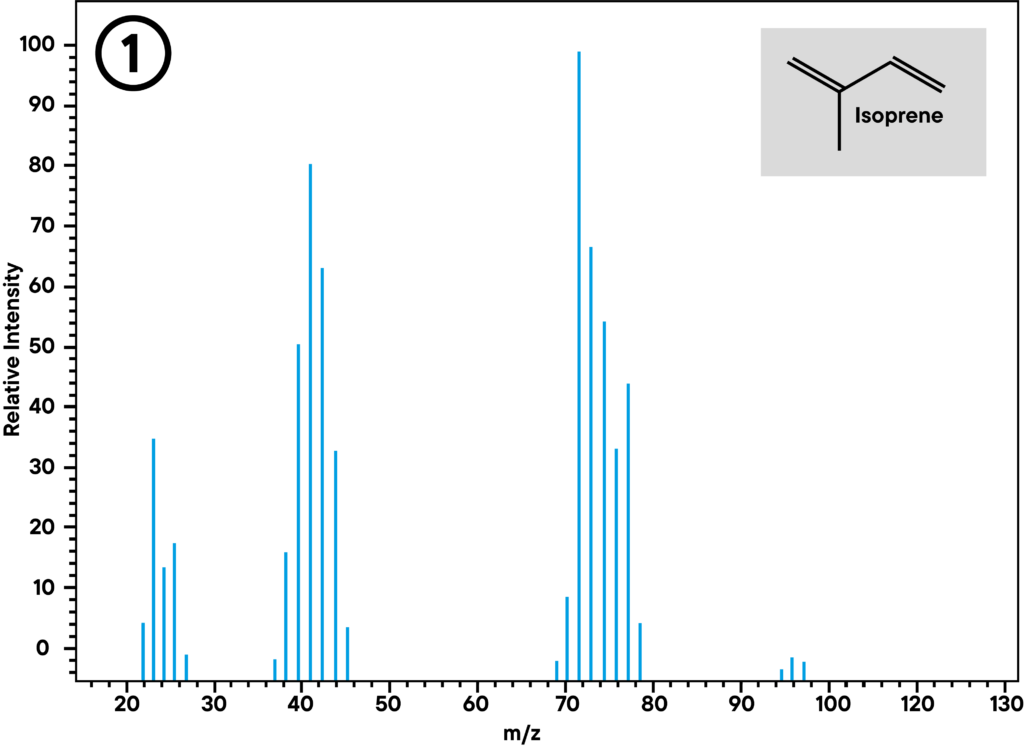
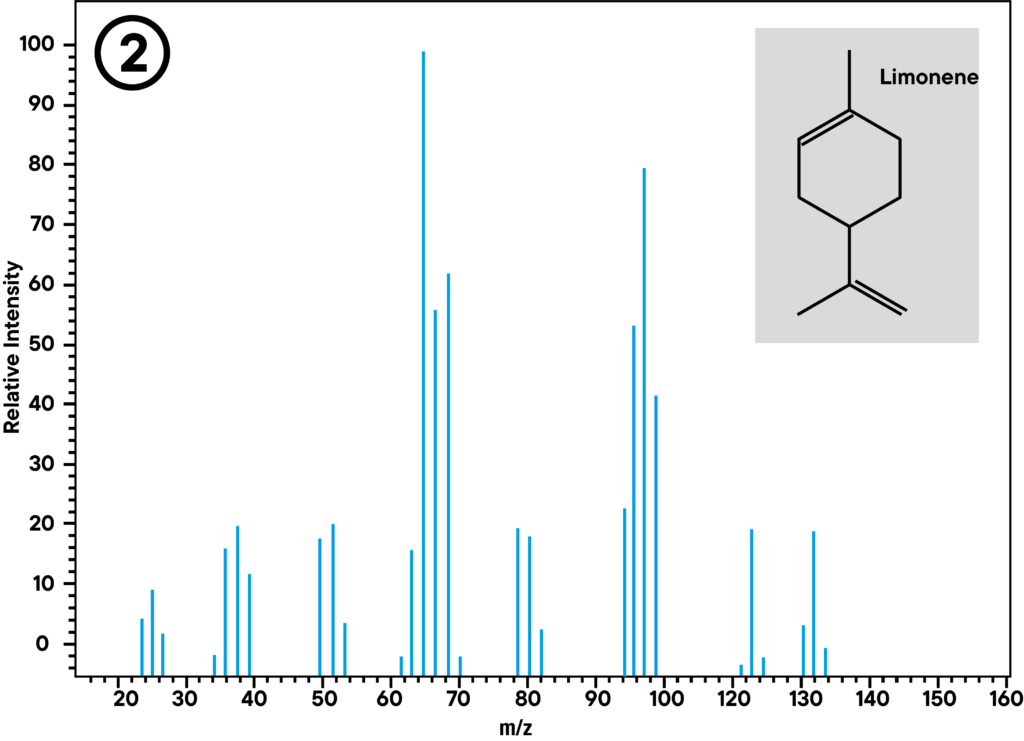
Application example: Thermal decomposition of latex
At 370 °C, synthetic rubber decomposes into several monomer parts. The main parts limonene and isoprene can be identified with STA in combination with GC-MS. The STA signal shows mass loss and enthalpy change at 372 °C. At the same time, GC shows two peaks, a smaller one (1) and a larger one (2), where the smaller one can be identified as isoprene and the larger one as limonene by mass spectrometry.
Well informed



