Table of Contents
Introduction
The direct reduction of iron ore with hydrogen is central to the decarbonization of the steel industry. Hydrogen-based processes enable a significant reduction of CO₂ emissions compared to conventional reduction with carbon carriers. High technical challenges related to gas reactivity, temperature control, pellet characteristics, and pressure conditions make experimentally validated kinetic data a key resource for the development of industrial H₂ direct reduction reactors. LINSEIS TGA and STA systems provide highly precise measurement data on reaction pathways, intermediate phases, and atmosphere dynamics—essential information for optimizing and modeling hydrogen reduction (Kim et al., 2021; Ratzker et al., 2025).
Reaction Chemistry and Process Fundamentals
The reduction of iron(III) oxide (Fe₂O₃) with hydrogen proceeds stepwise via Fe₃O₄ and FeO to metallic iron. The rate and efficiency of these conversions are influenced by numerous factors, including porosity, defects in pellets, diffusion properties, and atmosphere changes. Diffusion processes and hydrogen partial pressure largely determine reaction rates, while water vapor formed during hydrogen reduction must be continuously removed as a reaction product to avoid re-oxidation (Shankar et al., 2025; Fradet et al., 2023). Simultaneous analytical recording of mass changes, thermal effects, and gas phases is therefore essential for a complete understanding of the process.
Equipment Setup and Measurement Methodology
The LINSEIS TGA L87 MSB is particularly suitable for investigating powder samples and reference materials due to its high sensitivity. Rapidly switchable atmosphere control (including H₂, N₂, Ar, and their mixtures) allows for controlled varying conditions. Coupling with a mass spectrometer (MS) enables real-time analysis of formed gases, especially H₂O and potential by-products.
The LINSEIS STA L81 combines thermogravimetry (TG) and differential scanning calorimetry (DSC), so that during the reduction reaction not only weight changes but also energetic effects such as endothermic or exothermic reactions can be clearly assigned. Especially during the transition from Fe₃O₄ to FeO or FeO to Fe, characteristic thermal signatures occur that support the interpretation of reaction kinetics and intermediate phases.
The LINSEIS STA HP L85 enables measurements under real process conditions up to high hydrogen pressure and precisely controllable gas flows. This allows simulation of processes on complete pellets; pressure- and gas flow-dependent kinetics can be mapped, gas changes performed under load, and safety-relevant gas controls tested. The flexible selection of sample holders (platinum stirrup for powder versus ceramic crucible for pellets) complements the adaptability to different investigation designs.
Experimental Objectives and Evaluation Strategy
With these measurement platforms, the following scientifically relevant questions can be practically addressed:
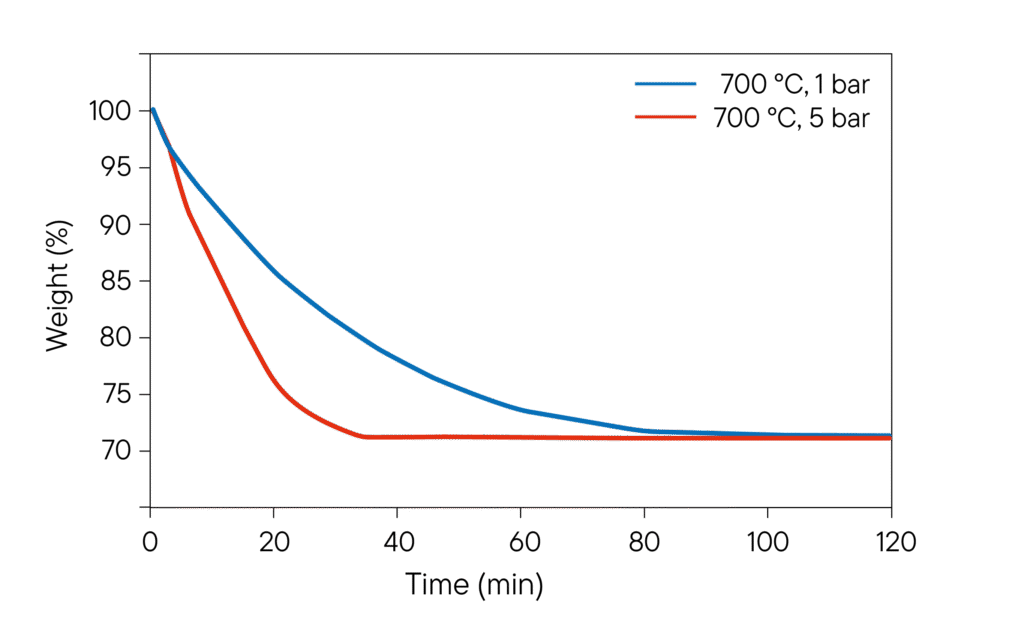
- Temperature-dependent reduction kinetics: Investigation at 600, 700 and 900 °C, differentiation of reaction rates for different pellet and powder forms.
- Pressure dependence: Series of experiments at 1, 10, 30 and 50 bar; identification of pressure influence on time to complete reduction.
- Intermediate phases and energetics: Step-by-step analysis of mass losses (Fe₂O₃ → Fe₃O₄ → FeO → Fe) and assignment of characteristic thermal effects via DSC.
- Gas phase analysis: Real-time detection of reaction products via MS, correlation between mass loss and hydrogen/water gas evolution.
- Microstructure changes: Before/after observation using electron microscopy (e.g., changes in pore structure and grain growth as a function of pressure and temperature).
- Combined modeling: Derivation of kinetic parameters that serve as a database for simulation-supported process optimization and scale-up (Raabe, 2021; Fradet et al., 2023).
Application and Industrial Perspective
The data sets generated by Linseis systems are essential for process simulation and for the development of control strategies in H₂-based direct reduction plants. They form the basis for quality assurance of pellets, help identify operating windows and safety limits, and allow modeling of complex gas phenomena in a wide variety of industrial applications (Souza Filho et al., 2021; Ratzker et al., 2025).
In addition to the following example on copper oxidation kinetics, the Forced-Flow concept can be seamlessly extended to pressure-controlled environments and reduction-driven reaction pathways, enabling a broader operational envelope for advanced gas–solid studies.
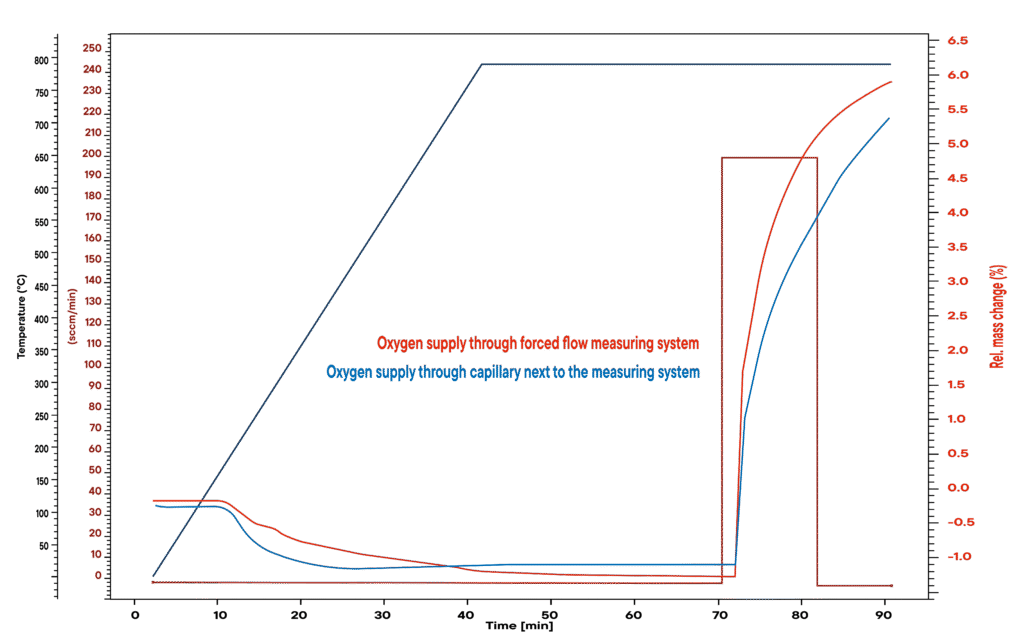
The oxidation of copper produces copper oxide, whereby the reaction rate is highly dependent on the gas supply. The forced flow principle ensures that the oxidizing agent (O₂) is distributed quickly and evenly over the entire sample material right from the start. This allows the reaction to take place much faster than with conventional methods, where the gas only reaches the sample gradually.
The reaction for the formation of copper oxide is:
2Cu + O₂ → 2 CuO
Due to the forced gas flow, the oxygen reacts efficiently with the copper – for accelerated reactions and more precise analyses under realistic conditions.
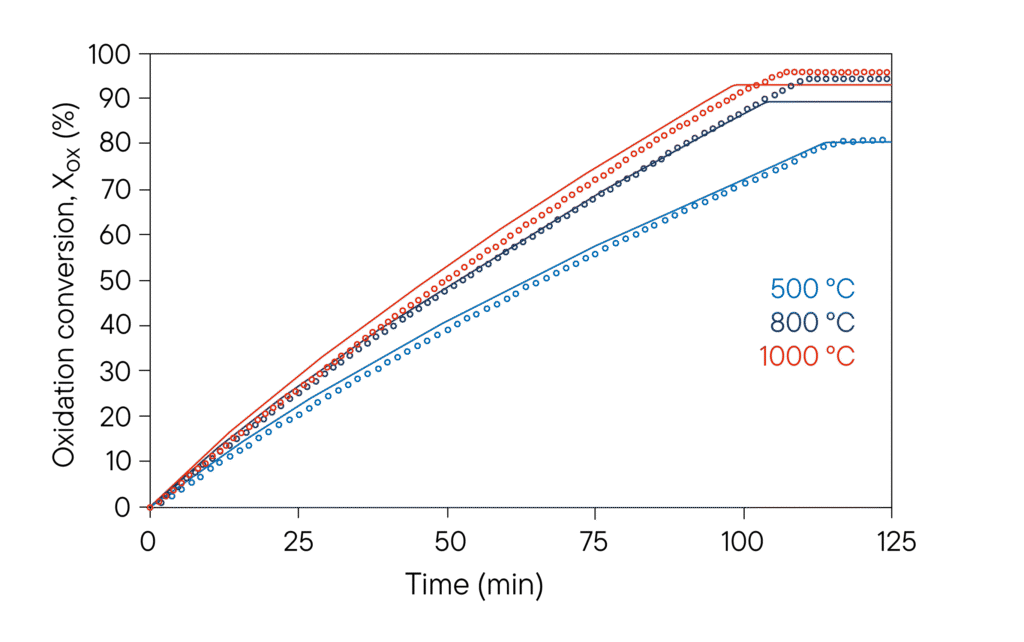
Conversion vs time curves obtained from macro TG iron ore air oxidation experiments (carried out in a Linseis TGA L83) at 500, 800 and 1000 ◦C (grey, black and red lines, respectively), using crucibles sealed with an alumina perforated lid (0.10 porosity) in all cases. Dots correspond to experimental results and continuous lines represent model predictions.
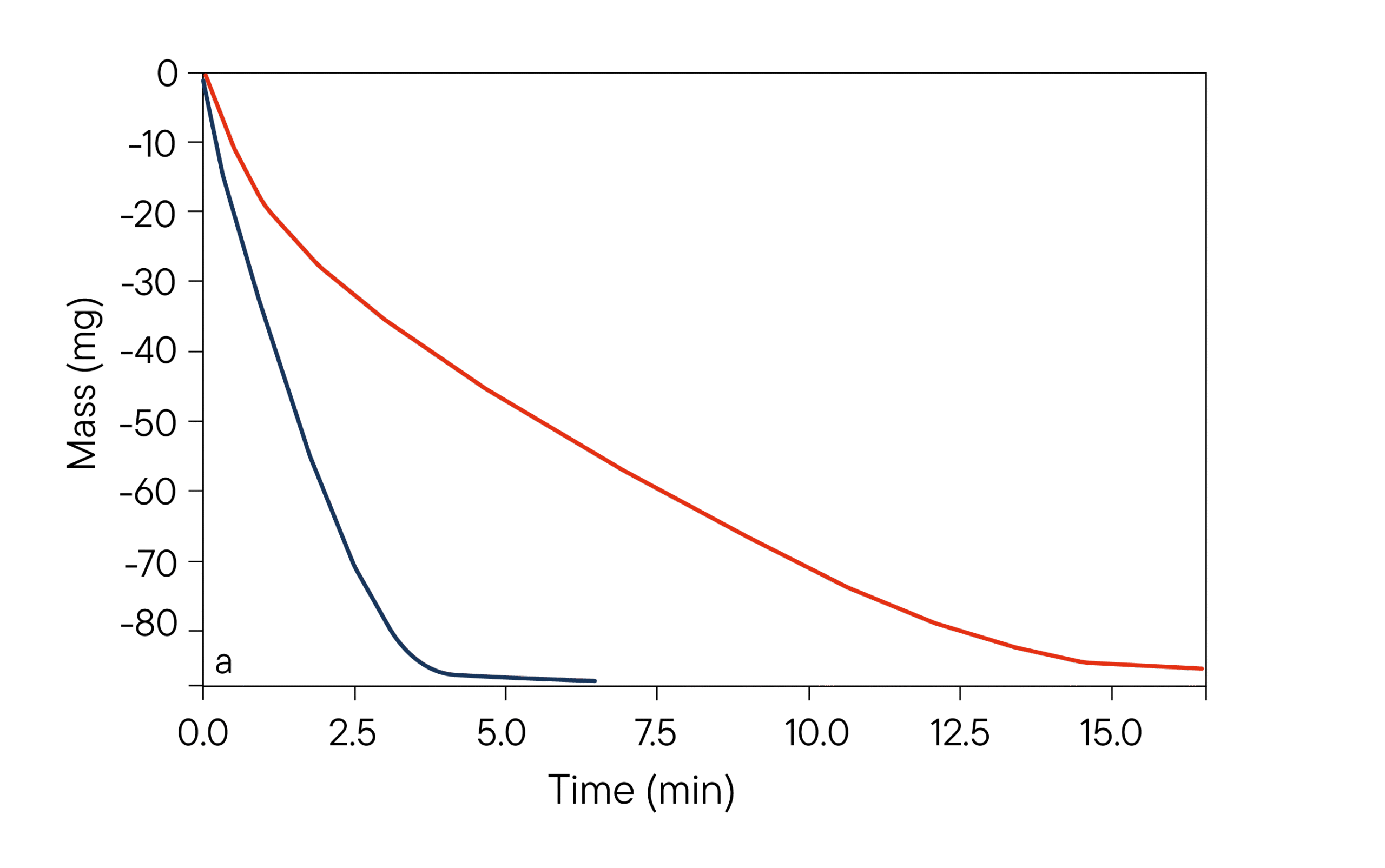
(a) Time dependent mass signal of the TGA
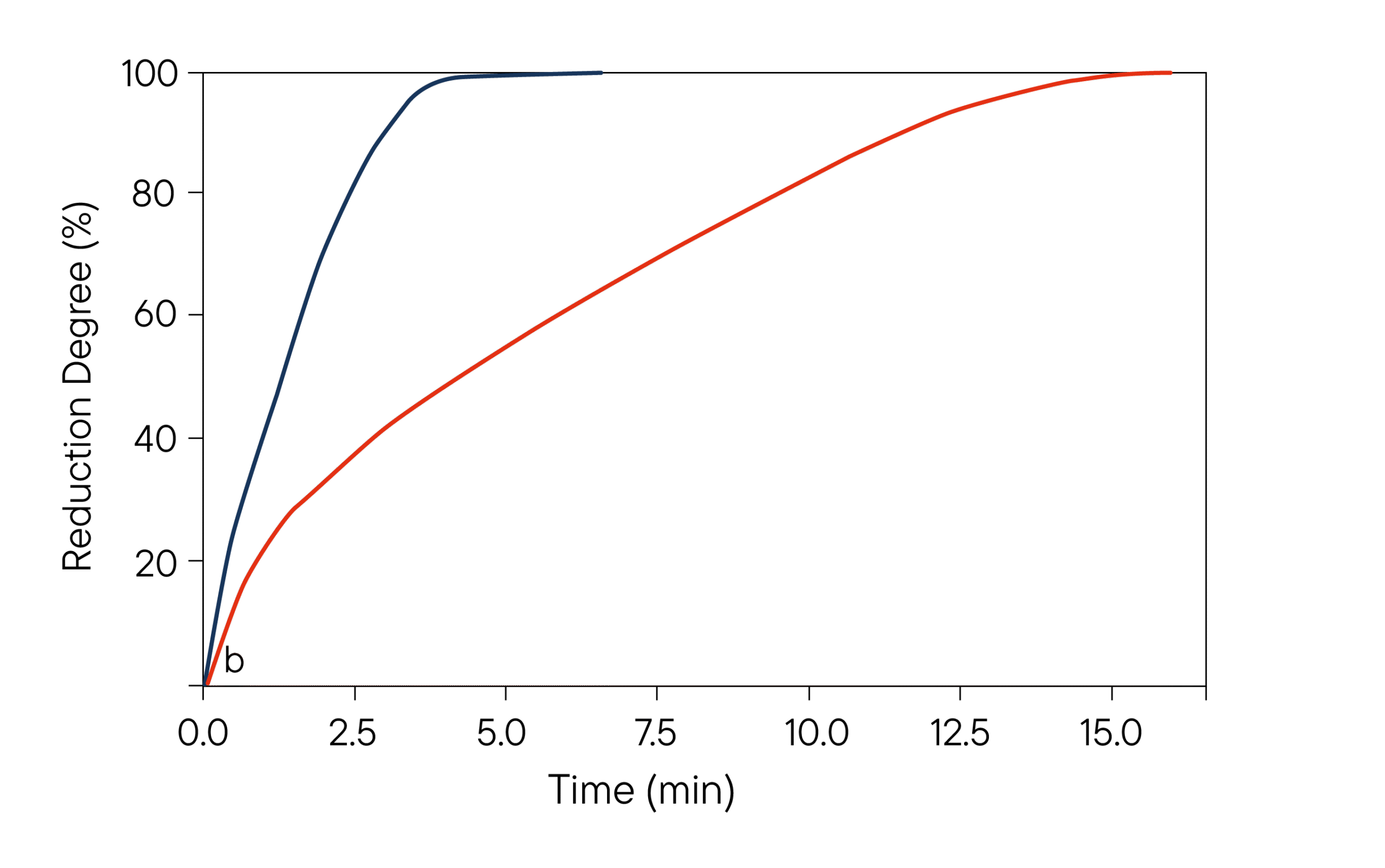
(b) Reduction degree (0e100%) as a function of time
Conclusion and Outlook
LINSEIS TGA and STA devices enable a unique combination of sensitivity, temperature and pressure stability, rapid gas change control, and flexible atmosphere selection. They are equally suitable for basic thermodynamic studies and for application-oriented process tests on powders and pellets. The future perspective includes the evaluation of complex gas mixtures (e.g., H₂/CO/CO₂) and the investigation of hydrogen cycles for future, fully sustainable steel processes (Ma et al., 2022).
References
Fradet, Q., Kurnatowska, M., & Riedel, U. (2023). Thermochemical reduction of iron oxide powders with hydrogen: Review of selected thermal analysis studies. Thermochimica Acta, 725, 179552. https://doi.org/10.1016/j.tca.2023.179552
Kim, S.-H., Zhang, X., Ma, Y., Souza Filho, I. R., Schweinar, K., Angenendt, K., Vogel, D., Stephenson, L., El-Zoka, A., Mianroodi, J. R., Rohwerder, M., Gault, B., & Raabe, D. (2021). Influence of microstructure and atomic-scale chemistry on the direct reduction of iron ore with hydrogen at 700 °C. Acta Materialia, 212, 116933. https://doi.org/10.1016/j.actamat.2021.116933
Ma, Y., Souza Filho, I. R., Zhang, X., Nandy, S., Barriobero-Vila, P., Requena, G., Vogel, D., Rohwerder, M., Ponge, D., Springer, H., & Raabe, D. (2022). Hydrogen-based direct reduction of iron oxide at 700 °C: Heterogeneity at pellet and microstructure scales. International Journal of Minerals, Metallurgy and Materials, 29(10), 1901–1907. https://doi.org/10.1007/s12613-022-2440-5
Raabe, D. (2021). Simulation of hydrogen-based direct reduction. Dierk Raabe Research. https://www.dierk-raabe.com/simulation-of-hydrogen-based-direct-reduction/
Ratzker, B., Ruffino, M., Shankar, S., Raabe, D., & Ma, Y. (2025). Elucidating the microstructure evolution during hydrogen-based direct reduction via a case study of single crystal hematite. Acta Materialia, 294, 121174. https://doi.org/10.1016/j.actamat.2025.121174
Shankar, S., Ratzker, B., da Silva, A. K., Schwarz, T. M., Brouwer, H., Gault, B., Ma, Y., & Raabe, D. (2025). Unraveling the thermodynamics and mechanism behind the lowering of direct reduction temperatures in oxide mixtures. Acta Materialia, 282, 120445. https://doi.org/10.1016/j.actamat.2025.05358 (also available as arXiv preprint arXiv:2504.12947)
Souza Filho, I. R., Ma, Y., Kulse, M., Ponge, D., Gault, B., Springer, H., & Raabe, D. (2021). Sustainable steel through hydrogen plasma reduction of iron ore: Process, kinetics, microstructure, chemistry. Acta Materialia, 213, 116971. https://doi.org/10.1016/j.actamat.2021.116971