Table of Contents
Heat storage for high-temperature processes
In the course of industrial decarbonization, the efficient use of thermal energy is increasingly becoming the focus of energy technology. Particularly in the area of concentrating solar power plants (CSP) and in the metalworking industry, there is a considerable need to store high temperatures (>600 °C) for hours or days – both to smooth out fluctuating energy sources and to recover industrial waste heat. In metal processing, for example, the waste heat generated during heat treatment can be temporarily stored in storage materials and later reused to preheat materials or in drying processes.
Heat accumulators are used for this purpose, which absorb thermal energy either sensitively (via temperature increase), latently (via phase change) or chemically (via reversible reactions). High-temperature applications are particularly demanding, as they require storage materials that remain mechanically, thermally and chemically stable – over several hundred charging and discharging cycles. The main challenge is to identify materials whose heat storage capacity remains constant over many cycles.
Particular attention is paid to solids such as graphite, ceramic insulators or composite systems consisting of these components. Such materials offer a wide range of applications as heat carriers, structural materials or matrices for other functional phases (e.g. salts, oxides). However, their performance cannot be assessed by chemical composition or melting points alone – the long-term behavior under cyclic thermal stress is decisive.
For the systematic evaluation of these properties, material characterization uses Differential Scanning Calorimetry (DSC) is used in material characterization. As a thermal analysis method, it enables the exact determination of heat capacity, transition temperatures and enthalpy changes over repeated temperature cycles. DSC is therefore an indispensable tool for analyzing material systems with regard to their cycle strength and thermal stability in the high-temperature range.
Recent studies show that targeted material combinations – such as ceramic-graphitic composites – can be used to develop systems that exhibit constant thermal performance despite high loads over hundreds of cycles (Yang et al., 2025; Ran et al., 2020). This article highlights the requirements for such heat storage materials, presents relevant material systems and shows how DSC contributes to the evaluation of their suitability for use.
Requirements for high-temperature heat accumulators
High-temperature heat accumulators must meet complex requirements in order to be used reliably on an industrial scale. Unlike storage tanks for low or medium temperatures, such as those used in building services, the main requirements here are thermal load capacity, chemical resistance and mechanical integrity over many cycles. The choice of material is significantly influenced by these multi-criteria decisions.
Thermal requirements
The ability to efficiently absorb and release thermal energy is key. In the case of sensible heat storage, this is achieved by increasing the temperature of a material, with the specific heat capacity (cₚ) determining the amount of energy stored. For high-temperature applications, materials are required whose cₚ values remain as constant as possible over the entire temperature range. A high absolute heat capacity is desirable, but it is more important that it does not drop over many charging cycles – an aspect that can only be clearly assessed through repeated measurements.
Thermal conductivity also plays a decisive role: materials with low conductivity cannot distribute heat evenly throughout the volume, which leads to unwanted temperature gradients and material stresses. The integration of highly conductive components – such as graphite – can make a targeted contribution to homogenizing the temperature distribution.
Chemical and mechanical stability
Thermal accumulators in industrial high-temperature applications are often exposed not only to heat, but also to reactive atmospheres, pressure differences or material contact with metallic, oxidizing or corrosive media. Resistance to chemical reactions is therefore a basic requirement. Oxidation, hydrolysis or the formation of unstable intermediate phases can lead to the gradual degradation of the storage capacity.
One example: graphite oxidizes in an oxygen atmosphere from around 600 °C – which limits its use in many applications without protective measures. Ceramics, on the other hand, especially those based on SiC or Si₃N₄, develop protective SiO₂ layers at high temperatures, which act as a diffusion barrier and prevent the penetration of oxygen.
Mechanical stability is also crucial. Repeated heating and cooling processes lead to thermal expansion and contraction, which generate stresses in the material. Materials with low thermal expansion and high fracture toughness have an advantage here. Ceramics offer excellent dimensional stability, while flexible, porous structures such as expanded graphite can partially absorb material stresses.
Evaluation by differential scanning calorimetry (DSC)
The above-mentioned requirements cannot be recorded using material data sheets alone. Only cyclical thermal analyses – such as those carried out with DSC – reveal how cₚ, enthalpy or phase transitions change in real operation. Several heating/cooling cycles are specifically simulated in DSC measurements. Deviations in the resulting calorimetry curves indicate a drop in performance or structural changes at an early stage.
DSC is one of the few methods that can simultaneously record these multi-physical changes, particularly in the case of new material combinations such as composite systems made of ceramics, graphite and salts. Studies such as that of Yang et al. (2025) or Ran et al. (2020) show that DSC can be used to make reliable statements about the thermal reversibility and stability of material systems – an essential prerequisite for the development of durable heat storage systems.
Graphite as heat storage and matrix material
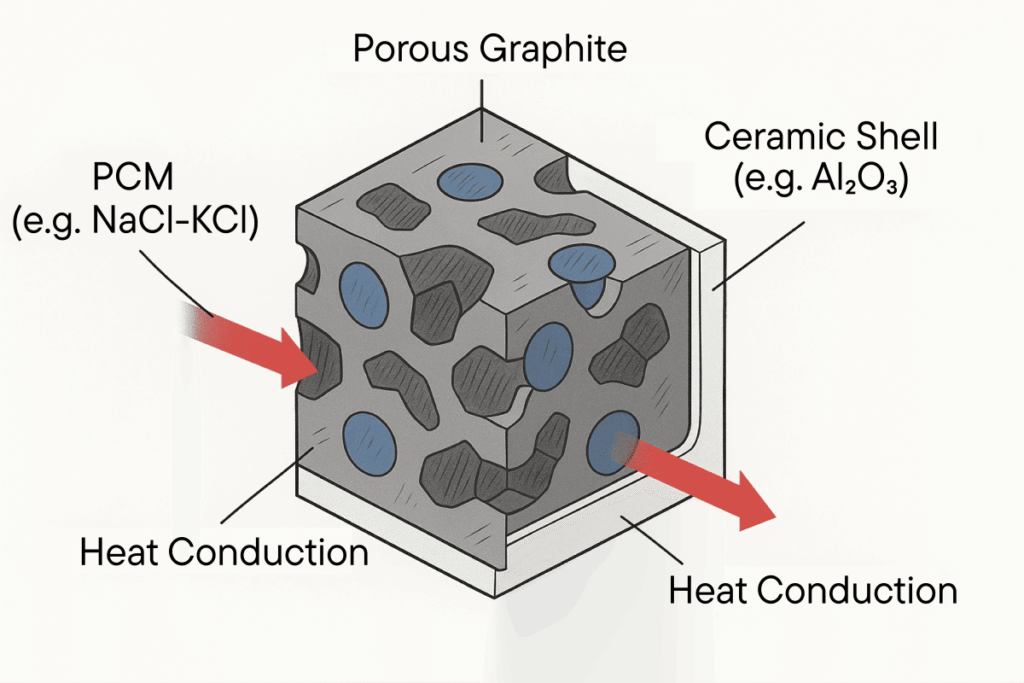
Graphite is one of the most studied materials for heat storage in the high temperature range – not only because of its thermal properties, but also because of its structural flexibility. In porous or expanded form, graphite can serve as a matrix material for other storage substances such as salts or metal oxides, while at the same time contributing to heat distribution and structural stability .
Thermal conductivity and temperature behavior
A key feature of graphite is its pronounced anisotropic thermal conductivity, which is significantly higher in the basal plane (parallel to the layer plane) than perpendicular to it. This enables effective lateral heat distribution, which is particularly advantageous in modular or layered storage systems. The specific heat capacity of graphite is moderate compared to other solids, but increases continuously with increasing temperature – a property that can be used for sensitive heat storage.
In operation, it has been shown that graphite remains thermally stable in an inert gas environment over many temperature cycles. Studies such as those by Yang et al. (2025) show that ceramically stabilized graphite composites maintain their storage capacity almost constantly over several hundred thermal cycles. The combination with ceramic materials protects the graphite against structural degradation and also has a temperature-stabilizing effect.
Susceptibility to oxidation and protective measures
In oxidizing atmospheres – especially in the presence of atmospheric oxygen – graphite begins to oxidize at temperatures of around 600°C. This severely limits its use in open systems. This severely restricts its use in open systems. In order to extend the application temperature ranges, passivating protective measures are often taken, for example:
- Operation in an inert gas atmosphere (argon, nitrogen)
- Embedding in ceramic cladding structures (e.g. Al₂O₃, SiC)
- Use of coating systems with diffusion-inhibiting properties
A practical example is the work of Ran et al. (2020)in which expanded graphite was combined with eutectic salts and ceramic additives. The composites not only showed improved thermal conductivity compared to the pure salt systems, but also significantly increased cycle stability. The role of the graphite here was both to absorb the salt and to improve the heat distribution in the volume. Thermal analysis using DSC showed that the stored enthalpy remained largely constant over dozens of cycles.
Application scenarios and material integration
In addition to its role as an active storage material, graphite can also serve as a structural carrier in more complex material composites. Particularly in module-based high-temperature storage systems, such as those used in CSP plants or industrial process heat systems, graphite can be used to create thermally conductive paths within an otherwise insulating system.
The integration of porous graphite structures also allows impregnation with PCM components or coupling with metallic storage media. Graphite acts as a shaping medium that combines thermal and mechanical functionality in one component.
Ceramic insulators: structure, protection and stability in high-temperature storage
Ceramic materials play a strategically important role in the context of thermal energy storage in the high-temperature range – not primarily as energy storage, but as structural, thermal and chemical stabilization components. They are used in the form of matrices, layers or functional embeddings and make a decisive contribution to the durability and safety of heat storage systems.
Thermal properties and application limits
Typical high-performance ceramics such as aluminium oxide (Al₂O₃), zirconium oxide (ZrO₂) or silicon carbide (SiC) are characterized by their extreme temperature resistance (>1500 °C), low thermal conductivity (typically <10 W/m-K) and very low thermal expansion. These properties make them ideal as thermal insulators in modular storage units, particularly for separating heat-conducting and heat-storing areas or for shielding sensitive materials.
The low thermal conductivity counteracts unwanted heat dissipation to the environment, while the high dimensional stability ensures mechanical integrity over many cycles. Under repeated thermal stress – as is typical in the charging/discharging operation of high-temperature storage tanks – these materials show no relevant structural changes.
Chemical stability: passivation and diffusion barrier
Another advantage of ceramic insulators is their chemical inertness to oxidizing, corrosive or reactive media. This is particularly relevant when used in combination with materials such as graphite, which oxidizes on contact with oxygen above 600 °C. Under such conditions, ceramics such as SiC or Si₃N₄ form passivating silicon oxide layers (SiO₂) on their surface. These act as a diffusion barrier against oxygen, which can also protect adjacent materials from oxidation.
In composite systems, such ceramics therefore take on a dual function: on the one hand, they act as a mechanical support structure and, on the other, as a chemically inert shell that shields graphite cores or PCM components from environmental influences, for example. This creates a controlled microenvironment that significantly extends the service life of the entire system.
Structural function in composite materials
Ceramics can be structured in a targeted manner – for example in the form of porous carrier materials, plates, honeycombs or bulk solids – and thus enable a precise design of the heat flow in the storage tank. In conjunction with thermally conductive components such as graphite, hybrid systems are created in which the advantages of both materials are functionally combined: mechanical resistance and chemical stability on the part of the ceramic, heat distribution and energy storage on the part of the graphite.
A successful example is provided by the work of Ran et al. (2020)in which ceramic components were embedded in a salt-graphite system. The ceramic matrix ensured an even distribution of the storage material, reduced thermomechanical stresses and at the same time improved the oxidation resistance of the entire composite body. The long-term stability was confirmed by DSC measurements over many temperature cycles.
| Graphite | 0.7–1.0 | >100 | High | Low (oxidation-prone) |
| Aluminum oxide (Al₂O₃) | 0.8–1.1 | <10 | High | High |
| Ceramic–graphite composite | variable | medium to high | High | adaptable (via composition) |
Differential scanning calorimetry (DSC): the key to evaluating cycle stability
The development of cycle-stable heat storage materials for the high-temperature range depends on reliable analysis methods that precisely quantify thermal properties. Differential scanning calorimetry (DSC) has established itself as one of the central test methods in this respect. It makes it possible to determine phase transitions, enthalpy changes and the specific heat capacity (cₚ) of materials as a function of temperature and over repeated load cycles.
Principle of the DSC
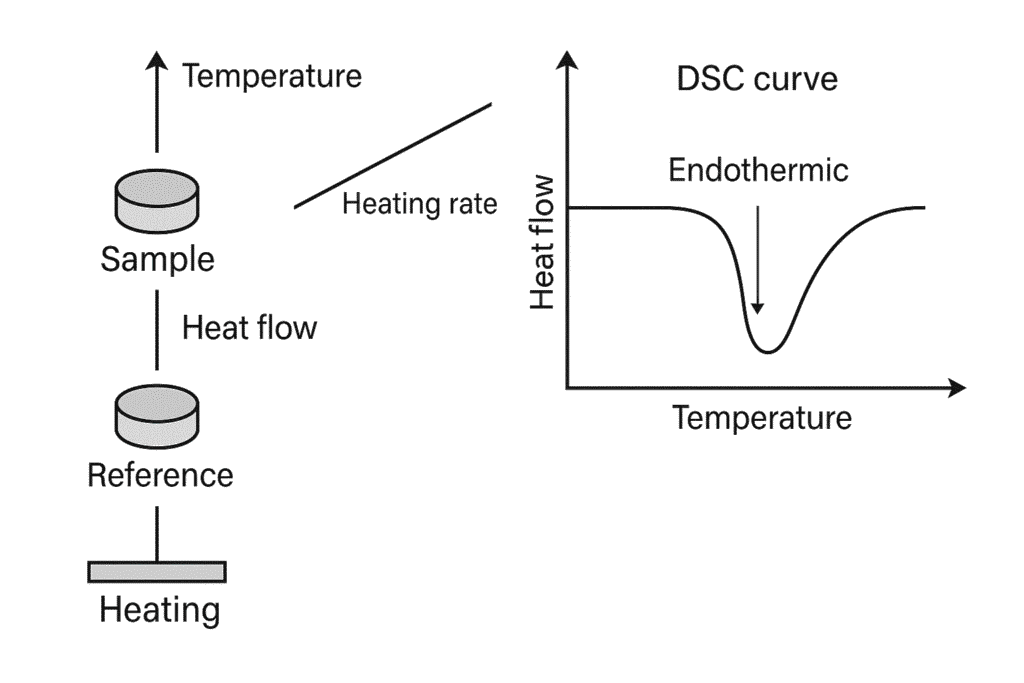
DSC measures the difference in heat flows between a sample and a reference while both are heated or cooled to a defined temperature in a controlled manner. Changes in the heat flow indicate physical or chemical transitions in the sample, for example:
- Endothermic processes: e.g. melting, phase change
- Exothermic processes: e.g. crystallization, reactions
- Temperature-dependent cₚ changes
How these thermal properties change over many cycles is particularly interesting for the evaluation of high-temperature heat accumulators. This is precisely where the strength of DSC lies: by repeating heating/cooling cycles, it is possible to determine whether and how quickly a material loses performance – for example due to structural changes, oxidation or phase separation.
Application on high-temperature materials
For materials such as graphite, ceramic-graphite composites or PCM-containing composites, DSC can be used to analyze key parameters such as heat capacity and transition temperatures not only in the fresh state, but also after many thermal cycles. For example, it is possible to see whether the stored enthalpy decreases over time or whether the temperature range in which a phase transition occurs shifts.
In the work of Yang et al. (2025) ceramic stabilized graphite composites were tested in several heating/cooling cycles. The DSC results showed stable thermal performance over several hundred cycles, with no significant drift in heat capacity or melting behavior. Such results not only prove the suitability of the material, but also the validity of DSC as a test method.
A similar approach can be found in Ran et al. (2020)which analyzed a eutectic salt-graphite-ceramic matrix. Here too, DSC was used to test the reversibility of the thermal transitions over repeated temperature stress – with positive results in terms of cycle strength.
Significance and limits
The advantages of DSC in material screening lie in:
- High sensitivity to small thermal effects
- Cycle-capable test protocols for simulating real storage loads
- Quantitative determination of heat capacity and enthalpy
- Wide temperature applicability (up to >1500 °C depending on the device)
At the same time, there are limitations: Measurement inaccuracies can occur at extremely high temperatures or with very large samples, as well as with highly anisotropic materials with high thermal conductivity. In such cases, a combination with other methods – such as thermogravimetry (TG) or dilatometric measurements – makes sense.
Conclusion and outlook: Systematically evaluating heat storage
Targeted heat storage in the high-temperature range is a key issue for industrial processes and renewable energy systems. In applications such as concentrating solar power (CSP) or the metalworking industry, highly efficient storage solutions can help to reduce energy losses, cushion peak loads and provide process heat in line with demand.
The analysis shows: Neither graphite nor ceramic materials meet all requirements in isolation. However, their combination in composite materials allows thermal conductivity, storage capacity and chemical stability to be combined in a targeted manner. Ceramics offer structural strength and chemical protection, while graphite efficiently distributes and stores heat as a matrix or additive.
Cycle stability is central to material selection: a heat accumulator is only suitable for practical use if it delivers constant performance over many charging and discharging processes. Differential scanning calorimetry (DSC) makes a decisive contribution here: it makes performance drops visible at an early stage, quantifies relevant characteristic values such as heat capacity and enthalpy, and allows the direct comparison of different material systems under realistic conditions.
The works cited by Yang et al. (2025) and Ran et al. (2020) show how highly stable storage materials can be developed through targeted material combinations and precise analysis. These findings are increasingly being incorporated into material development for industrial storage solutions.
Perspectives
Future developments will focus on the following aspects:
- Scalability and production of cost-optimized composite materials
- Standardized test methods for comparable evaluation of cycle stability
- Long-term tests under real operating conditions
- Combination of DSC with other analysis methods (e.g. TG, X-ray diffractometry)
With a view to industrial implementation, it is clear that materials science can make a significant contribution to increasing the efficiency, durability and operational reliability of thermal storage systems with systematic analysis such as DSC. This makes it an integral part of sustainable energy systems – from laboratory scale to industrial scale.
References
- Yang, X. et al. (2025): Self-heating ceramic-graphite composites with stable thermal energy storage capacity, ACS Energy Letters, 10(3), 1234-1242. DOI: 10.1021/acsenergylett.4c03270
- Ran, X., Wang, H., Zhong, Y., Zhang, F., Lin, J., Zou, H., Dai, Z., & An, B. (2021). Thermal properties of eutectic salts/ceramics/expanded graphite composite phase change materials for high-temperature thermal energy storage. Solar Energy Materials and Solar Cells, 231, 111047. DOI: 1016/j.solmat.2021.111047