Melting describes the phase transition of a substance from a solid state to a liquid state [1, chapter 2.5.7]. During the melting process, the temperature of the substance remains constant. This means that the heat introduced into a DSC by the furnace or heater is used to convert the sample into the liquid state. The energy required for this is called the enthalpy of fusion and is abbreviated with the formula symbol ∆HM. If a sample melts during a DSC measurement, this forms a peak in the direction of endothermic. Since the specific melting enthalpy of a substance is considered for the identification or evaluation of samples, in practice the results are usually automatically divided by the sample mass.
Therefore, it is assumed in the following that determined enthalpies have already been divided by the sample weight. If this process is not superimposed by other processes of the sample, the baseline returns to the value before phase transformation after the melting process. By integrating this peak area, the enthalpy ∆HM is determined. Depending on the shape of the peak and the DSC signal before and after the peak, there are different methods of evaluation to determine as accurate a representation as possible of the energy actually recorded. If a mixture of substances is present, the mixing ratios can be determined by separate evaluation and by using the literature values for melting enthalpies of the corresponding substances. If the two melting peaks of the substances are very close to each other, double peaks or shouldered peaks can occur. These peaks, which merge into each other or are superimposed, must be evaluated separately in order to be able to make reliable statements. Two examples of such peaks are listed and evaluated below (Fig. 1) with relevant temperatures.
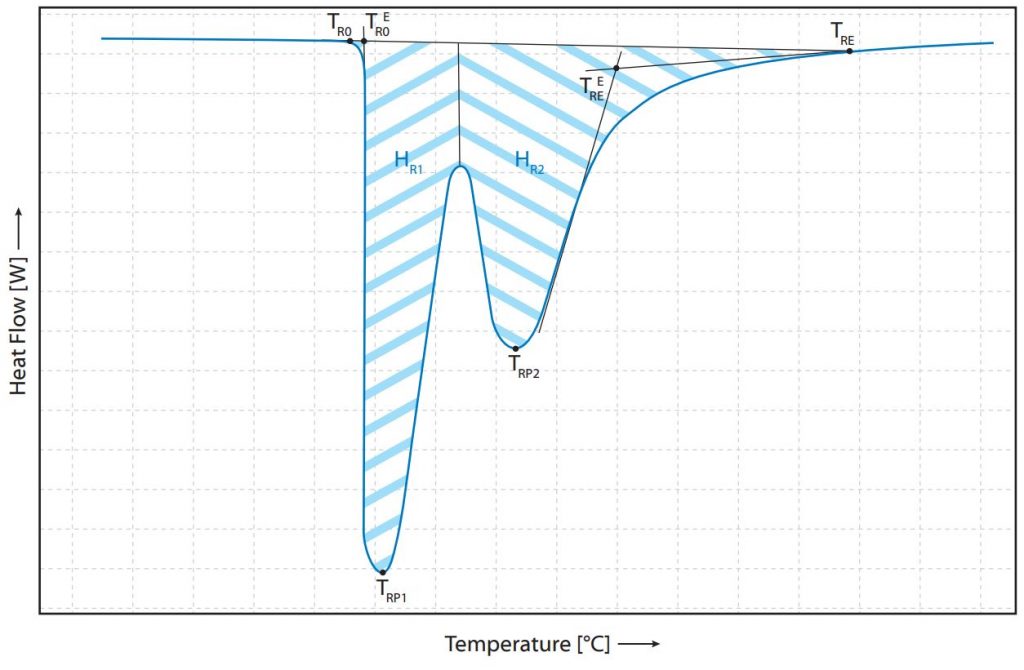
Literature:
- B. Wunderlich, Thermal Analysis of Polymeric Materials. Berlin, Heidelberg: Springer-Verlag Berlin Heidelberg, 2005.
- Prüfung von Kunststoffen und Elastomeren Thermische Analyse Dynamische Differenzkalorimetrie (DDK), DIN 53965, 1994.
