A gel is a colloidal system that consists of a continuous solid phase enclosing or interspersed with a liquid phase (often water). It behaves as a solid and is elastic despite being mostly liquid. Gels are formed by the cross-linking of polymers or colloidal particles that form a three-dimensional network trapping the liquid.
Gels are non-Newtonian fluids, which means that their flow behavior does not follow the laws of classical Newtonian viscosity. Unlike Newtonian fluids, whose viscosity remains constant, non-Newtonian fluids such as gels respond differently to shear forces. Their viscosity can change under mechanical stress, such as pressure or stretching. This means that gels exhibit viscous properties when deformed slowly, but tend to behave in a solid or elastic manner when subjected to rapid or strong stress.
Gels exist in different types and shapes, frequently used in pharmaceuticals or as an adhesive. A special version of gels are so called xerogels, as the swelling agents are usually removed. Examples for a xerogel would be dried silica gel or gelatin. Gels can be easily characterized by DSC, as it is frequently done in quality control.
App. Nr. 02-011-012 Chip-DSC 100 (Chip-DSC L66 Ultimate) – Xerogel nanoparticles
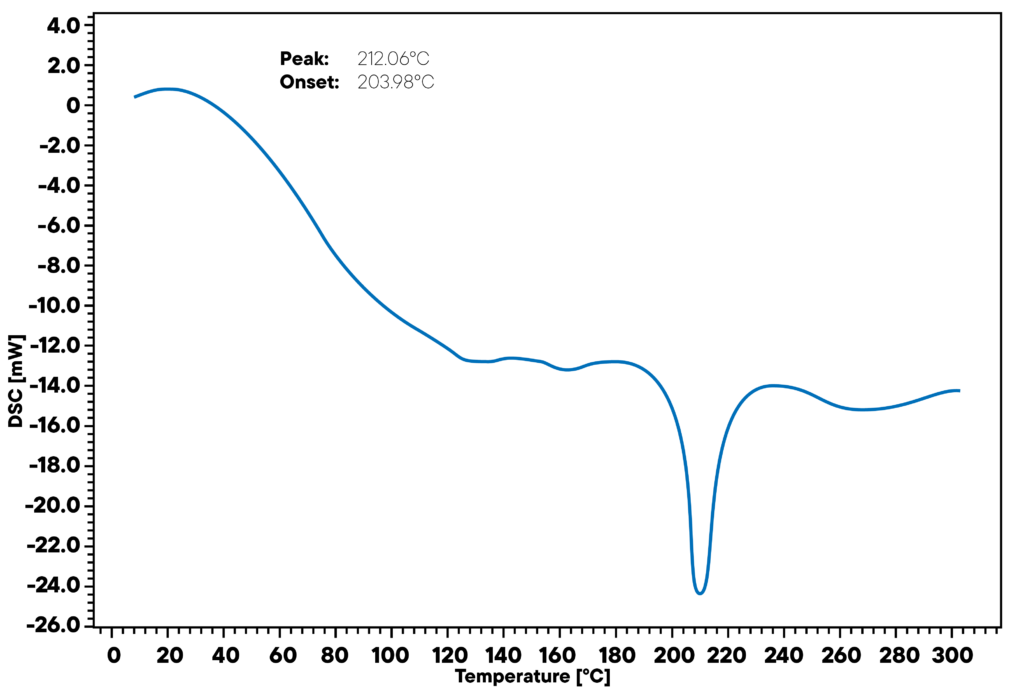
The curve shows a linear DSC run of Alumina Nano Particles in Gel Matrix, heated with linear heating rate of 10K/min in nitrogen atmosphere. The signal shows two significant effects during the run that are worth closer consideration:
There is a loss of water in the range up to 120°C, leading to a shift in baseline due to the Cp change that is caused by the mass change of the sample. As a consequence of this effect, a dry gel matrix containing the nanoparticles is remaining – the so called xerogel.
At around 200°C, there is a phase transition of the nanoparticles from ordered to amorphous Alumina structure that can be seen as a small sharp peak. These two effects are reproducible and characterize the nanoparticle gel well.

