Differential Scanning Calorimetry
What is differential scanning calorimetry analysis?
The Differential Scanning Calorimetry (DSC) is main techniques of thermal analysis. DSC detects endothermic and exothermic transitions like the determination of transformation temperatures and enthalpy of solids and liquids as a function of temperature.
In contrast to classic calorimetry, where a sample is put into an isolated chamber to monitor its heat uptake and release in an isothermal experiment, the differential scanning calorimetry is a dynamic procedure.
A typical calorimeter is an isolated chamber where a sample is placed in a surrounding medium. Then the sample is heated with a definite amount of heat. The difference in temperature between sample and surrounding medium gives the heat capacity of the sample and information about heat release and consumption of the sample. Besides that, the differential scanning technique uses a sample and reference that are facing the same conditions and their signal is directly subtracted from each other.
Where is Differential Scanning Calorimetry used?
DSC analysis is used for many applications in a broad range of industries like polymers as well as for fundamental research in academia. Examples include glass transition determination and the investigation of chemical reactions, melting and crystallization behavior.
Other DSC applications deal with the influence of additives, fillers or the processing of materials. The characteristic shape of the individual DSC curves is also used for quality control applications.
What is the difference between DTA and DSC?
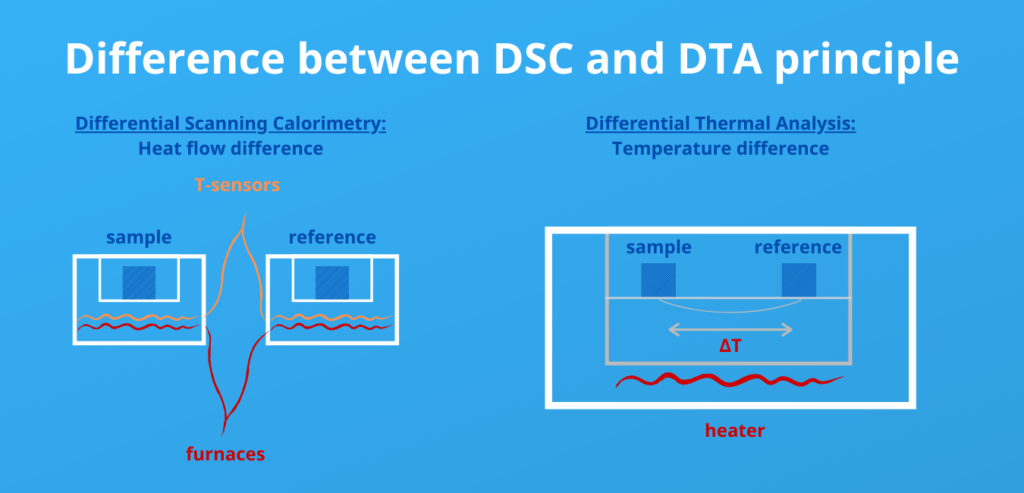
The difference between DSC and DTA is: DSC measures heat flow difference and DTA measures temperature differences between a reference sample and a sample of interest.
Optical dilatometry – black and white image during a heating process
What is the difference between a heat flux DSC-analysis and a power-compensated DSC-analysis?
DSC devices are built according to two basic measuring principles: the heat-flux principle and the power-compensated DSC principle.
With the Heat-Flux DSC, the changes in the heat flow are calculated by integrating the ΔTref curve. For this type of DSC analysis, a sample and a reference crucible are placed on a sample holder with integrated temperature sensors to measure the temperature of the crucibles. This arrangement is located in a temperature controlled furnace. In contrast to the classical design, the characteristic feature of this DSC is the vertical arrangement of planar temperature sensors surrounding a planar heater. This arrangement allows a very compact, lightweight and low heat capacity structure with the full functionality of a DSC furnace.
In power-compensated DSC-analysis, the sample and reference crucible are separated in space. Then the temperature of both chambers is controlled so that the same temperature is always present on both sides. Instead of the temperature difference between the two crucibles, the electrical power required to reach and maintain this state is then recorded.
What are the standards for differential scanning calorimetry?
- DIN 53765: Testing of plastics and elastomeres; thermal analysis; DSC-method
- DIN 51007:2019-04: Thermal analysis – Differential thermal analysis (DTA) and differential scanning calorimetry (DSC) – General Principles
- DIN EN 6041:2018-03: Aerospace series – Non-metallic materials – Test method – Analysis of non-metallic materials (uncured) by Differential Scanning Calorimetry (DSC); German and English version EN 6041:2018
- DIN EN 728: Plastics piping and ducting systems – Polyolefin pipes and fittings – Determination of oxidation induction time; German version EN 728:1997
- DIN EN ISO 11357-1, -2, -3, -4 and -6: Plastics – Differential scanning calorimetry (DSC) – Part 1: General principles (ISO/FDIS 11357-1:2016); German and English version FprEN ISO 11357-1:2016
- ASTM E 126: Standard Test Method for Inspection, Calibration, and Verification of ASTM Hydrometers
- ASTM E 1356: Standard Test Method for Assignment of the Glass Transition Temperatures by Differential Scanning Calorimetry
