In this study, results of TG-DSC measurements on calcium nitrate tetrahydrate – Ca(NO3)2. 4H2O- are presented and discussed. This salt is already widely used as a material for heat storage and heat transfer as it is inexpensive and extremely effective.
The sample was analyzed using a Linseis STA PT 1000 instrument, which simultaneously monitors the weight change and the DSC signal. The enthalpy of the phase transitions and the heat capacity can be determined from the DSC signal.
The sample was heated in closed aluminum crucibles to 180 °C at a heating rate of 10 K/min and kept isothermal for 3 hours. It was then heated to 600 °C at a heating rate of 10 K/min.
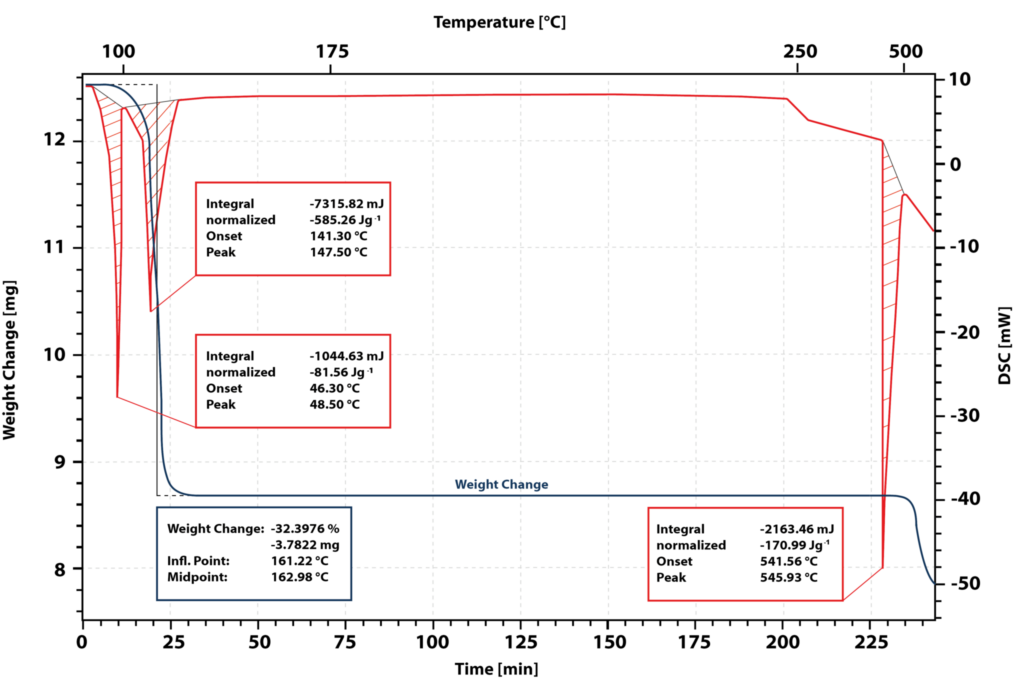
The blue curve shows the mass loss and the red curve the DSC signal. The first peak in the DSC signal is caused by the melting of the sample. The beginning of the melting peak is at 46 °C.
After the sample has completely melted, a second endothermic peak with an onset at 141 °C occurs. The TG signal shows a weight loss of 32 % in this temperature range. The water of crystallization of the calcium nitrate tetrahydrate is split off and solid anhydrous salt is formed.
During the isothermal holding time at 180 °C, the sample undergoes no further changes, which means that this temperature is ideal for drying the salt and obtaining the anhydrous salt.
When heated again to 541 °C, an endothermic peak occurs. The anhydrous salt melts. However, the TG signal shows a loss of weight. This indicates that the salt decomposes during melting. Therefore, the enthalpy of fusion and the heat capacity of the molten anhydrous salt cannot be measured directly.
However, this can be achieved by further TG-DSC measurements of salt mixtures. The calcium nitrate must be mixed with lithium, sodium or potassium nitrate in different molar percentages. The enthalpies of fusion can be determined from the DSC melting peaks of the mixtures. The enthalpy of fusion of the pure calcium nitrate can then be calculated simply by extrapolation to a molar percentage of 100 % based on the calcium nitrate.
The same method is used to measure the heat capacity of the molten anhydrous calcium nitrate.

