Oxidation induction time
What does the OIT measurement indicate?
This simple standard test procedure characterizes the oxidation stability of oils, fats, fuels, or polymers. This information is important because external influences such as light, chemical or biological substances, as well as radiation and temperature, can strongly alter the physical properties of a material.
If an oxidizing agent is present during a measurement, it can lead to the oxidation of the sample [2, Chap. 2]. In this process, electrons are transferred from the substance under consideration to the available oxygen, forming an oxide as a reaction product.
Oxidative behavior of a sample
Oxidation is an exothermic process, the onset of which is reflected in a DSC signal as an increase. A comprehensive view of the entire oxidation process, which forms an exothermic peak on the DSC signal, is rarely captured. To assess the pre-conditioning and resistance of substances, it is possible to make statements about the oxidative behavior of a sample using DSC measurement.
Difference between OIT and OOT
For oxidation reactions, there are various standards to ensure comparable analysis. These standards provide uniform measurement conditions for consistent results.
Oxidation Onset Temperature Measurement (OOT)
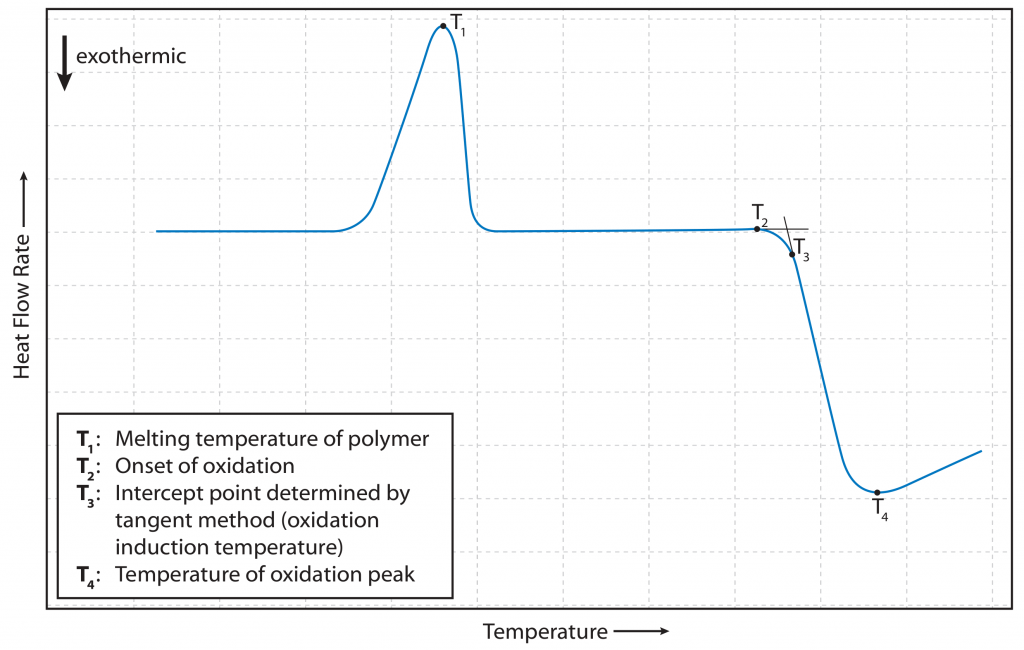
The first of these oxidation tests is the Oxidation Onset Temperature Measurement. In this test, a static atmosphere is used where the oxidizing agent, oxygen, is already present. The sample is then heated at a defined heating rate. The temperature at which the DSC signal exhibits a significant inflection point due to oxidation is the OOT.
The evaluation is performed by drawing two tangents to the DSC curve before and after this inflection point. The corresponding intersection point represents the onset temperature sought. The method is illustrated in Fig. 1.
Figure 1: Illustration of an oxidation onset temperature measurement [3, p. 8]
Oxidation-Induction-Time (OIT)
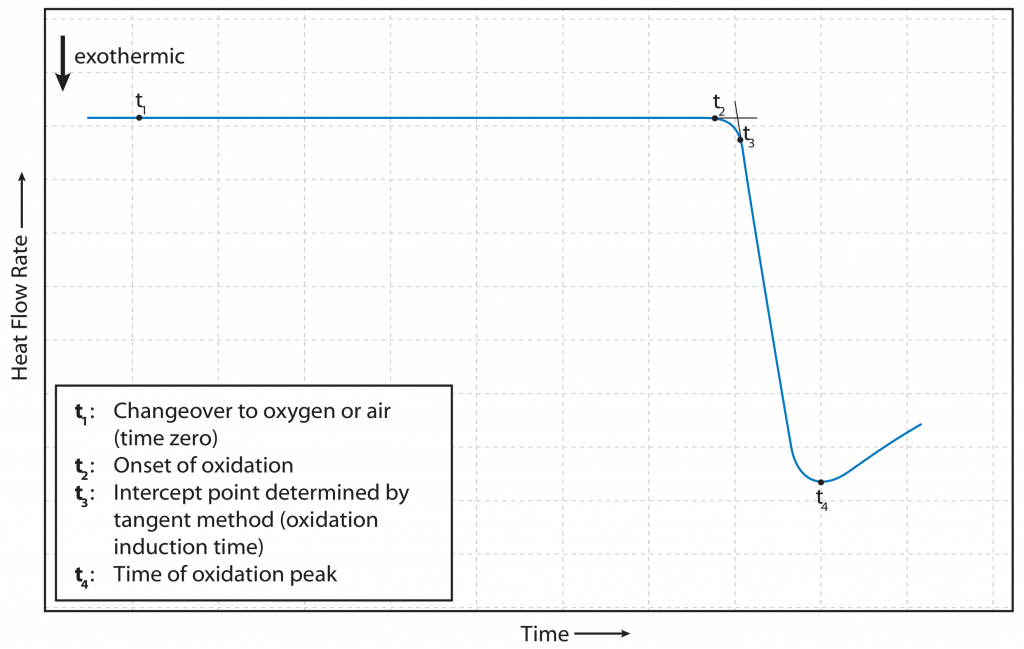
The second examination for oxidation stability is the Oxidation-Induction-Time, illustrated in Fig. 2. In this test, the sample is subjected to inert conditions, i.e., free from reactive agents, at a defined temperature below the OOT and maintained.
At a certain point in time, the oxidizing agent is then introduced into the sample chamber. The time from the introduction of the oxidizing agent to the onset of oxidation on the sample, also characterized by the inflection point of the DSC signal, is the OIT. The evaluation is analogous to OOT but dependent on time.
Figure 2: Illustration of an oxidation-induction time measurement [3, p. 7]
The static and dynamic OIT methods
To ensure economical and reproducible measurements for highly oxidation-stable compounds, high-pressure DSCs are used in practice. The OIT is shortened depending on the pressure. Due to the higher oxygen concentration resulting from better heat and oxidant exchange, the result is more pronounced with a steeper change in slope.
There are two standard OIT methods: Firstly, the dynamic procedure, in which the sample is continuously heated in an oxygen-containing atmosphere, and oxidation is observed as a change in the curve. Both the temperature and the duration from the onset of oxidation are measured.
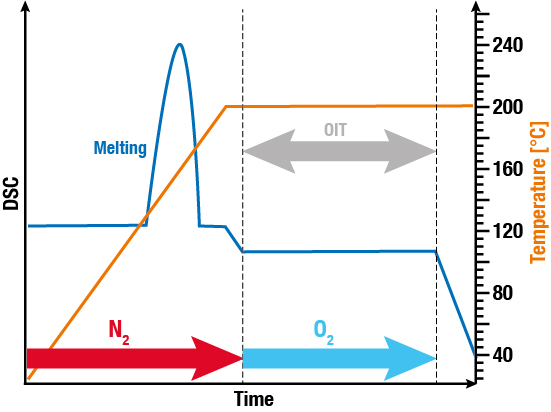
On the other hand, there is the static method, where the sample is heated in a protective atmosphere, typically nitrogen, to 190 – 220 °C. After reaching a constant temperature, oxygen or air is introduced into the chamber. Subsequently, the duration between the introduction of oxygen/air and the onset of oxidation (exothermic effect) is measured.
Decomposition during Oxidation
Decomposition refers to the breakdown of a compound into smaller molecules or elements [1, Chap. 3.4.4]. If there is no oxidizing agent present during a measurement, such as atmospheric oxygen, pyrolysis occurs at a temperature dependent on the substance. The substance system is split and decomposed by the input of heat. In a DSC, this can be achieved by using an inert sample gas such as nitrogen, as oxidation can otherwise occur and influence this process. Decomposition is an endothermic process.
Sources
[1] B. Wunderlich, Thermal Analysis of Polymeric Materials. Berlin, Heidelberg: Springer-Verlag Berlin Heidelberg, 2005.
[2] G. W. Ehrenstein, G. Riedel und P. Trawiel, Thermal analysis of plastics: Theory and practice. Munich: Hanser, 2004.
[3] Plastics – Differential Scanning Calorimetry (DSC), ISO 11357-6, 2008.