-
-
Thermal Interface Materials – Heat Spreaders
15. April 2024Heat spreaders are thermally conductive objects that act as a bridge between a heat source and a heat exchanger.
-
Glass transition temperature
5. April 2024The Glass Transition Temperature Tg, also known as the glass point, is a point on the temperature scale at…
-
3D printing with metals – Thermal analyses
2. April 2024Among the methods of 3D printing with metals, Direct Laser Metal Sintering (DMLS), also known as Laser Power Bed…
-
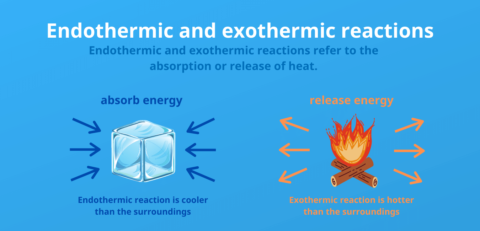
Endothermic vs. exothermic reactions
2. April 2024Endothermic reactions absorb energy in the form of heat from their surroundings, which leads to the surroundings becoming colder.
-
Molten Salts – Heat transfer of the future
7. February 2024Molten salts are stable at high temperatures above 300 °C and exhibit remarkable thermal properties.
-
Acrylonitrile butadiene styrene (ABS): An in-depth look
9. January 2024Acrylonitrile-Butadiene-Styrene, commonly known as ABS, is a copolymer and a central component in various industrial and commercial sectors.
-
Polyvinyl chloride (PVC): An in-depth investigation
18. December 2023Polyvinyl chloride, also known as PVC, is a versatile thermoplastic that plays a significant role in many industrial and…
-
Polystyrene (PS): A detailed analysis
11. December 2023Polystyrene, also known as Polystyrol, is a widely used polymer produced through the polymerization of styrene.
-
Low density polyethylene (LDPE): A summary
6. December 2023Low density Polyethylene, LDPE, is a thermoplastic polymer made from the monomer ethylene. It is a highly branched plastic,…
-
High-density polyethylene (HDPE): An inside look
1. December 2023High-Density Polyethylene (HDPE) and Polyethylene (PE) are both types of Polyethylene, but they exhibit different properties due to variations…
-
Polyamides: An Overview
1. December 2023Polyamides are essential protagonists in the world of polymer science and technology. Among the many polyamides are types such…
-
Polyethyleneterephthalate (PET): An in-depth investigation
13. November 2023Polyethylene Terephthalate (PET), commonly known as PET, holds a significant place in various industrial and commercial productions.
This thermoplastic reveals… -
-
-
Deformation techniques of metals
4. November 2023The deformation of metals is a pivotal process in modern industry, encompassing a wide array of applications from automotive…
-
Raman spectroscopy with DSC
30. October 2023Raman spectroscopy is a technique for studying molecules and determining their structure and dynamics. It uses excitation-induced scattering of…
-
Phase change analysis with DSC
9. October 2023Materials can be categorized and described in different ways. This can be done, for example, based on their external…
-
DC and AC Hall Effect Measurements
26. April 2023The investigation of the Hall effect of a material is specially used for the determination of the Hall coefficient…
-
Determination of the CTE density
1. March 2023How is the CTE density determined with thermal expansion? In the following article you can read more about thermal…
-
Thermal Impedance
1. March 2023In electronic devices the thermal management is a crucial performance factor since the overheating of components can lead to…
-
Analysis of thermoelectric materials – the figure of merit and its measurement
17. January 2023Thermoelectric materials can convert heat directly into electrical energy. This property is based on the Seebeck effect, in which…
-
3D Printing Polymers
29. July 2022Injection molding, often also referred to as injection molding or injection molding process, which is based on primary molding….
-
Thermal insulation and thermal insulation materials
19. July 2022Whenever objects with different temperatures are in physical contact or are in the range of radiation influence they undergo…
-
Biomass
14. July 2022Biomass is plant-based or animal-based material used as fuel to produce heat or electricity.
The most common biomass materials used… -
PCM – Phase Change Material
6. July 2022The term “phase change material” (PCM) usually describes materials that are used as so-called latent heat storage.
-
Thermal contact resistance
15. June 2022The thermal contact resistance characterizes the transfer of heat at the interface between two solids.
-
3D printing in ceramics
30. May 2022Ceramics are used in many areas of industry. As a rule, ceramics are first formed as green bodies from…
-
Specific heat capacity
3. May 2022“Specific heat capacity indicates the ability of a substance to store heat. This substance quantity corresponds to the amount…
-
Hydrogen Storage Solutions
29. March 2022As the mass specific energy density of hydrogen (33.3 kWh/kg) is one of the highest of all fuels, the…
Linseis Messgeraete GmbH
Vielitzerstr. 43
95100 Selb / Germany
Tel.: +49 (0) 9287/880 0
Fax: +49 (0) 9287/704 88
Mail: [email protected]
USA
Linseis Inc.
109 North Gold Drive
Robbinsville, NJ 08691
Tel.: +01 (609) 223 2070
Fax: +01 (609) 223 2074
Mail: [email protected]
©2024 Linseis GmbH