Thermal Analysis of Nuclear Materials
Ernest Rutherford was the one who postulated an atomic model that could explain the behavior of matter scientists had observed so far in 1911. It was clear that there was a kind of elemental “building kit” consisting of different sorts of smallest particles, so-called atoms that were able to interact with each other, to form bigger agglomerations, so-called molecules. He developed a model that described atoms as spherical particles that consist of positive charged protons, neutral neutrons and negative charged electrons. The protons and neutrons were also responsible for the mass of the atom as electrons almost had no mass. The mass containing protons and neutrons were located in the atomic core which was considerable small and the electrons were located in a hull with considerable big diameter (see Fig.4).
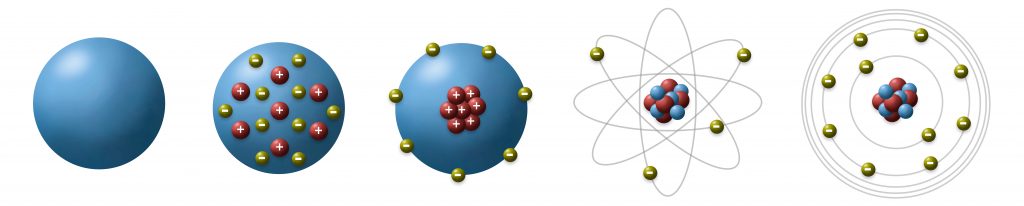
Fig.4: Development of the Rutherford atomic model: Assuming atoms to be spherical structures containing positive and negative particles he found that the negative charge carriers are located in the so-called atomic hull.
At present day we know that this elemental description of atoms is correct in general, besides the fact that the orbits for electrons are much more complex than Rutherford was postulating. The Rutherford model is still used at school to explain the fundamental setup of atoms and their nature. But Rutherford was not only postulating the atomic model but also, as mentioned, was able to explain the radioactivity, Curie and Becquerel had discovered. When radioactivity was discovered, atoms were thought to be the smallest existing particles. It was not clear that they were able to decompose into smaller atoms by sending out radiation, energy and particles. However, the fact that they were sending out particles and radiation looked logical if they were considered to be structured in a way Rutherford had postulated (see Fig.5). The three types of radiation that were known were from then on classified as alpha-radiation (particles are send out), beta-radiation (electrons are send out) and gamma-radiation (electromagnetic energy is send out). Later, the particle irradiation was separated in alpha-irradiation (alpha particles / He-cores) and neutron emission, while gamma-irradiation was separated into x-rays and y-rays depending on the wavelength. We meanwhile know that radioactive radiation is caused by nuclear fission, either by statistic fission of radioactive materials or by chain reaction or fusion reaction.
Fig.5: Radioavtive atoms undergo nuclear fission. The unstable atomic cores collapse into smaller cores by sending out particles, radiation and energy
Atoms that have high atomic masses (heavier than lead and bismuth) are not stable and their cores are decomposing into smaller atoms by emitting radiation. These elements that undergo statistic fission are called radioactive elements. What kind of radiation they emit is depending on their atomic mass and nature of their core structure. The different kinds of radiation are shown in Fig.6. As can be see, the different types of radiation are potentially penetrating matter which makes it hard to control it once the radiation is released. And depending on the sort of radiation, it can transform non-radioactive atoms into radioactive matter. If matter is exposed to radioactive radiation, it may change its structure by becoming radioactive itself. In case of life forms or humans it can have a devastating influence as radioactivity can’t be sensed biologically however is very dangerous by mutating cells or even burn organic matter.
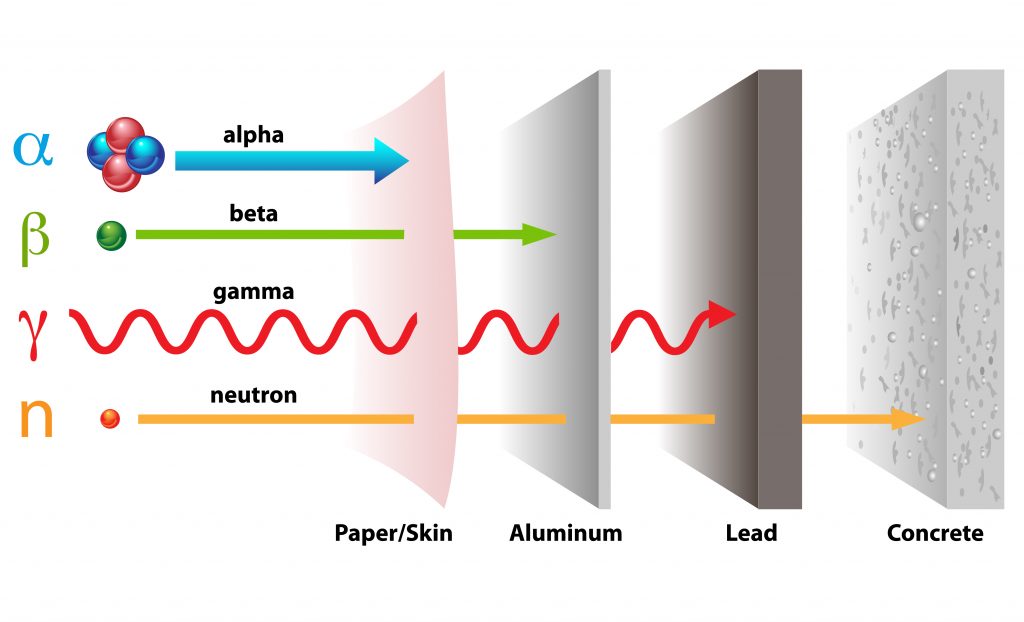
Fig.6: Different types of radioactive radiation and their potential of penetrating different types of solid matter
Industrial use of radioactivity
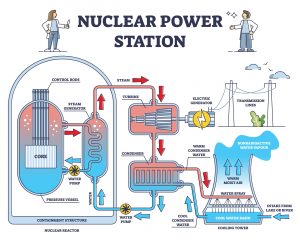
Fig.7: Typical setup of a nuclear reactor. Left: Core with nuclear fuel and two water loops; middle: turbine for power generation and condenser; right: cooling tower
As radioactivity was known since the beginning of the 20th century and a lot of scientists were working on this interesting new field, it took until 1938 when Otto Hahn and coworkers finally discovered the nuclear fission and possibility of controlled nuclear fission. In the following years, the second world war took place, leading to the fact that one of the first uses of this discovery was of military nature. But since the 1950s, nuclear energy is the most important energy source around the world. With its advantage of clean and cheap power supply, nuclear reactors were undergoing a continuous global improvement during the last 50 years. Meanwhile reactors of the 4th generation such as very high temperature reactors (VHTR) or sodium cooled fast reactors (MSR) are currently under development and will be the future for nuclear energy. The general setup of a core reactor is shown in fig.7: A setup of so-called nuclear fuel rods is placed in a chamber (in most cases in a water bath), creating heat. The heated water is transferred to a second water loop that powers a steam turbine system and is transferred back through a condenser. The condenser is coupled to environmental water that is used for cooling. This cooling water is what can be seen as white steam that is release from the big cooling towers that are iconic for nuclear power plants.
Nuclear fuel
The technical use of radioactivity is mainly based on the nuclear chain reaction, a concept where the fission of one radioactive core causes multiple fission events in neighbouring atoms by emitting neutrons. (see fig.8) Usually, uranium and its salts are used as neutron source. However, the natural uranium species are dominated by 98% of U238 which is not emitting much neutrons when undergoing fission. Therefore, the uranium U235 has to be accumulated first. This is a process where the 1% U235 species of natural uranium is separated in a complex process by gas centrifugation. The result is uranium with a much higher content of U235 that emits enough neutrons to start a chain reaction or a self-sustaining ongoing fission process. For nuclear reactors, a U235 content of at least 20 % is needed, while in weapon technique, the content has to be significantly higher. In a nuclear power plant, so-called fuel rods are made of accumulated uranium (see fig.9). These rods are positioned in array structures containing passive control rods and active reaction rods. The passive control rods consist of inactive material that stop the chain reaction by “catching” neutrons while the active rods are emitting neutrons and run the chain reaction cascade. When controlled properly, the reaction is in a stable condition and is self-sustaining, emitting constantly heat which creates steam for the turbine. Additionally, in most reactors, a mechanical robot system can exchange used active fuel rods and replace them by fresh ones. With that setup, the reactor can run permanently and create energy on demand, independent of any fossil fuels, wind or sunlight.

Fig.9: The nuclear fuel rods consist of Uranium filled pellets that are arranged in a stack (rod) and put into a matrix of multiple controlling and reaction rods.
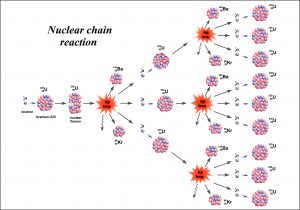
Fig.8: In most cases, U235 is used as power source. When hit by a neutron the core collapses and forms new species, releasing more neutrons that can react in a cascade with other U235 cores.
Drawbacks of nuclear energy
When used, the nuclear fuel rods usually undergo a recycling process where the still usable U235 species are separated and used for new fuel rods. All the other elements that are created during the chain reaction (like Pu, Po…) are also separated and used for other purposes such as clinical applications or other industrial processes. For some of these isotopes, a nuclear chain reaction is the only source that can even create them. However, a lot of material is just radioactive dead matter that does not see any other use than being stored as nuclear waste. These waste materials are emitting a lot of radiation and are very dangerous and toxic to the environment, so they have to be stored somewhere where they are inaccessible and do not influence ground water, natural resources or life. A proper place for that purpose is of course hard to find but still this is the only chance to get rid of atomic waste as there is no way to neutralize the radioactivity once the material is existing. Also, the nuclear reactors themselves have to be unmounted in a very long lasting and complex process once the reactor is abandoned, causing high cost and creating even more nuclear waste material. Of course, besides the waste topic, there is also the risk of accidents and unwanted release of nuclear material into the environment. This, socalled greatest possible accident, happened a few times in the past, having devastating influence on life on earth in 1986 in Chernobyl and 2011 in Fukushima.
Research
The nuclear power supply and industrial use of nuclear technology comes with a broad field of ongoing research. Not only the optimization of nuclear fuels and their recycling is a hot topic but also the work with radioactive samples for clinical research and therapy as well as further improvement for reactor safety and performance optimization. Scientists still are looking for storage and re-use technologies for nuclear wastes and also try to design nuclear fuel rods that produce only minimum amounts of toxic isotopes. Besides that, one of the biggest research projects is the development of fusion reactor technology that is promised to solve the worlds energy problems once it is available. The advantage of a fusion reactor would be an almost radiation-free energy generation that requires only small amounts of hydrogen to form helium in a fusion reaction.
Fig.11: The future nuclear power plant will be most likely not a fission but a fusion reactor. A massive setup of magnets can keep the plasma beam of billion degree hot hydrogen within a field that allows to control the fusion reaction of hydrogen cores into helium, resulting in huge amounts of released energy and only very little radiation and no toxic isotopes.
Thermal analysis in nuclear research

Fig.12: Research with radioactive material requires special environments. In most cases, socalled hot cell units are established where all the equipment has to be included to separate the “hot” area from the normal lab environment
Due to the research that is done in that field, there is a need of analytical equipment and especially also for instruments of thermal analysis. Of course, these special applications and safety requirements need a lot of modifications of the standard devices, which Linseis can do. As nuclear radiation may interfere with the electronics, this has to be separated from the mechanical parts of the instruments and must be put outside the hot cell or glove box. We did customizations of nearly every important instrument in thermal analysis that include the adaption for hot cell applications such as separation of electronics and control panels. Mainly dilatometers, thermo-balances as well as combined TG-DSC and also Laser Flash techniques were successfully transferred into a nuclear design by us in the recent past. Our experience in this special field makes Linseis become the worldwide leader in thermal analysis of nuclear materials as we are the most flexible player on that market.
Applications
Graphite standard
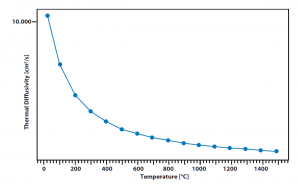
The curve shows the thermal diffusivity data of a graphite standard from NIST that was measured with a special LFA with fibre-optical connected laser and separated electronics. The results are matching with the literature values and accuracy and power of the laser are completely identical with the standard instrument.
Graphite is one of the most important materials in reactor construction and due to its high thermal conductivity and temperature stability it offers various purposes it can be used for.
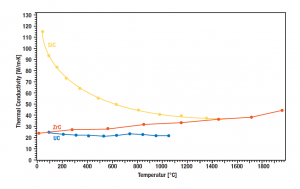
Thermal conductivity of carbides
Different types of carbides were measured by LFA to determine the thermal conductivity. Uranium carbide was compared to similar Zirconium carbide and classic silicon carbide. As a result, the uranium carbide showed a considerable low value of around 25 W/mK, while zirconium carbide shows an increasing trend that has an electronic dominant character, whereas the SiC shows a more or less common decrease shape of its thermal conductivity curve.
Downloads
Linseis Thermal Analysis for nuclear energy (PDF)